함께하는 세상, 풍요로운 내일을 위해
최선의 노력을 다하는 이원의료재단입니다.
최선의 노력을 다하는 이원의료재단입니다.
연구 분야
임상 검체 검사에서 축적된 노하우를 바탕으로
다양한 연구분야에 집중하고 있습니다.
체외진단용 의료기기 임상평가
이원의료재단 체외진단 의료기기 평가 서비스
-
풍부한 의료기기 임상시험 수행 노하우 보유
단계별 최적화 지원
-
국내 최대 수탁기관으로 다양한 검체 보유
다양한 임상 시험 가능
-
임상적 성능 평가 및 유효성 검사까지 One site
신속한 임상 시험 가능
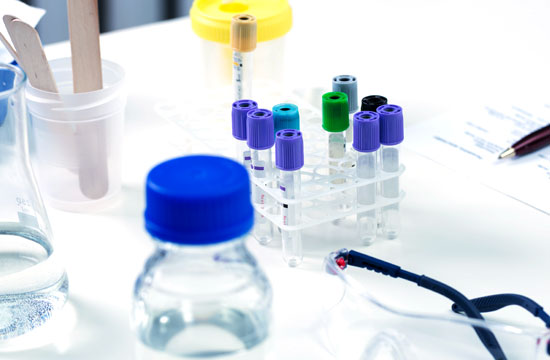
체외진단용 의료기기
인체에서 유래한 시료를 검체로 하여 검체 중의 물질을 검사하여 질병 진단,
예후 관찰, 혈액 또는 조직 적합성 판단 등의 정보 제공을 목적으로 체외에서
사용되는 시약을 통칭합니다. 다만, 실험실에서 조제하여 사용하는
조제시약은 제외됩니다.
의료기기의 제조(수입) 허가, 신고, 인증 대상
의료기기법 제6조제2항(제조업의 허가 등) 및 제15조제2항(수입업의 허가
등)에 따라 제조(수입)자는 제조 또는 수입하려는 의료기기에 대하여 허가,
신고, 인증 받아야하며, 대상은 다음과 같습니다.
- 1등급 의료기기 : 제조(수입) 신고
- 2등급 의료기기 : 제조(수입) 인증(허가)
- 3,4등급 의료기기 : 제조(수입) 허가

| 등급 | 체외진단용 의료기기 분류기준 |
|---|---|
| 1등급 | 개인과 공중보건에 미치는 잠재적 위해성이 낮은 경우 |
| 2등급 | 개인에게 중증도의 잠재적 위해성을 가지며 공중보건에 미치는 잠재적 위해성이 낮은 경우 |
| 3등급 | 개인에게 고도의 잠재적 위해성을 가지며 공중보건의 중증도의 잠재적 위해성을 가지는 경우 |
| 4등급 | 개인과 공중보건에 고도의 위해성을 가지는 경우 |
허가 절차 흐름도
특화된 기술개발 지원시스템을 통하여 개발 아이디어에서부터
체외진단용 의료기기 평가 도출까지 신속˙정확한 결과 제공이 가능합니다.
-
01
의료기기 해당여부 확인
-
02
의료기기에 해당하는 경우
-
03
등급에 관한 규정에
따라 1,2,3,4 등급으로 분류
-
1등급
[신고]
-
2등급
[인증,허가]
-
3, 4등급
[허가]
의료기기 임상절차
주요 임상시험 용어
| NO | 영문 | 국문 | NO | 영문 | 국문 |
|---|---|---|---|---|---|
| 1 | Adverse Event | 이상반응 | 17 | Informed Consent Form | 시험대상자설명서 |
| 2 | Applicable Regulatory Requirement | 관련 규정 | 18 | Inspection | 실태조사 |
| 3 | Audit | 점검 | 19 | Institution | 임상시험실시기관 |
| 4 | Audit Certificate | 점검확인서 | 20 | Institutional Review Board | 임상시험심사위원회 |
| 5 | Case Report Form | 증례기록서 | 21 | Investigator | 시험자 |
| 6 | Clinical Trial/Study | 임상시험 | 22 | Monitor | 임상시험모니터요원 |
| 7 | Clinical Trial/Study Report | 임상시험 결과보고서 | 23 | Multicenter trial | 다기관임상시험 |
| 8 | Comparator | 대조기기 | 24 | Nonclinical Study | 비임상시험 |
| 9 | Comparator | 대조약 | 25 | Principal Investigator | 시험책임자 |
| 10 | Compliance | 임상시험의 준수 | 26 | Protocol | 임상시험계획서 |
| 11 | Confidentiality | 비밀보장 | 27 | Protocol Amendment | 임상시험 변경계획서 |
| 12 | Contract | 임상시험계약서 | 28 | Site Management Organization | 임상시험실시 지원기관 |
| 13 | Contract Research Organization | 임상시험수탁기관 | 29 | Source Document | 근거문서 |
| 14 | Coordinating Committee | 조정위원회 | 30 | Standard Operating Procedure | 표준작업지침서 |
| 15 | Essential Document | 임상시험 기본문서 | 31 | Subject/Trial Subject | 임상시험대상자 |
| 16 | Informed Consent | 대상자동의 | 32 | Subject/Trial Subject | 임상시험피험자 |